 |
Cooke Lab |
|
| |
RESEARCH> Fish Surgery |
|
| |
|
|
| |
Fish Surgery |
|
| |
 |
|
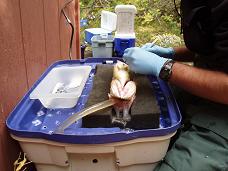
Fish surgeries are performed for the purpose of biotelemetry/biologging applications. Our lab takes the welfare of fish seriously as we want the data collected from the individual to be reflective of untagged conspecifics. Our procedures are documented and forwarded to the Institutional Animal Care Committee for approval prior to any form of work and/or studies that involve animals. We maintain a continuous commitment to improving practices/skills in our research and the safety/welfare of the fish that we work on. The following information outlines surgery practices adapted from Wagner & Cooke (2005) and Cooke et al. (2006, unpublished chapter on Attachment of Devices). |
|
|
| |
|
|
| |
When performing fish surgeries, it is recommended that the surgical tools and table to be used be sterile. This reduces the chance of disease and pathogen transfer among fishes between surgeries. Surgical tools can be sterilized by steam under pressure (autoclaving), through the use of a hot bead sterilizer, or with solutions such as Cidex (followed by rinsing with sterile saline). Multiple sets of surgical tools could also be prepared ahead of time and kept in sterile packets prior to use. In addition, using disposable sterile tools will minimize the amount of equipment required. New suture packages and a new pair of sterilized surgical gloves (standard in fish surgery to protect the fish and surgeon) should be used for each fish. However, standard non-sterile surgical gloves are also suitable when the surgeon is not touching the inside of the wound with their fingers.
Prior to surgery, fish are held in flow through tanks where they are anaesthetized (with either clove oil emulsified with ethanol (9:1) or MS-222). When a fish becomes unresponsive, it is then positioned on the surgery table in a supine position. The head is placed slightly lower than the rest of the body. This position allows the water to pool around the gills and also prevents water from entering the incision site. A pump is used to circulate aerated water over the gills. Sometimes a smaller maintenance dose of anesthetic is circulated over the gills during surgery.
To maintain a clean field, surgeons should avoid touching fish with their gloves during surgery, leaving the handling of fish to an assistant. Transmitters should be disinfected prior to implantation after consulting with the telemetry manufacturer. The incision site should be disinfected using a sterile saline solution and a cotton swab to remove excess mucous.
While performing surgery, water should be kept out of incision site and off of surgical tools, gloves, the transmitter and suture material. It is believed that water entering the peritoneal cavity of fish can promote bacterial growth and may result in death. The use of a clear sterile plastic drape can help maintain a clean surgical area and keep the incision site clear of water while keeping the rest of fish moist.
Once the animal is prepared for surgery, a decision regarding where the incision should be made by visualizing where the device are placed. It is important to make sure that the location of the incision will not be obstructed by bony structures such as the pelvic girdle. Incisions can be made easily if the fish scales are removed. This practice can cause additional trauma (since fish scales are epithelialized and embedded in the dermis) resulting in greater tissue damage when compared to fish without scale removal. Incisions must be made with extreme care and control to ensure the underlying viscera are not disturbed (since muscle color of the body wall tends to be similar to the viscera) when cutting through the body wall. Self-retaining retractors can be used to keep the coelom open and simple curved forceps with blunt tips can also be used to lift the body wall upward from the viscera. Incisions should always be made with a single or minimal possible number of cuts to prevent maceration of the tissue. The length of the incision should be sufficient for the transmitter to be inserted without touching the surrounding skin. Bleeding during surgery can be removed using sterile swabs/pads. Water should never be used to clean a wound where bleeding has been observed unless it is sterile saline solution.
Fish will always be exposed to stress as a result of the handling and anesthesia. They must be revived prior to being released back to their environment. This procedure requires fish to be held in a recovery tank for some time, to be monitored, until they are alert and responsive. |
|
|
| |
|
|
| |
|
|
| |
Anesthetics
Anesthetics are generally used on fish for the purpose of physiological studies, tagging, tissue sampling, transport and euthanasia. Anesthetics immobilize fish by depressing their central and peripheral nervous systems.
Fish should always be anesthetized when intraperitoneal implantation or laparotomy is conducted. The use of anesthetics for other procedures such as external and gastric implantation depends on the characteristics of the animal, the goal of the study and the type of attachment. These procedures are often performed without anesthetics. Rules and regulations on the types of anesthetics to be used on fish vary by country. Different jurisdictions have different requirements for the types of procedures that can be done without anesthesia. Therefore, it is essential to consult with local authorities (Institutional Animal Care Committees and federal bodies) and veterinary staff. |
|
|
| |
|
|
| |
Common types of anesthetics used for fish surgery include:
- tricaine methanesulfonate (MS-222)
- clove oil (main active ingredient is eugenol)
- effective alternative due to benefits such as its non-carcinogenic property, rapid induction, relatively short recovery time and low cost
Others include:
- benzocaine
- 2-phenoxy-ethanol
- metomidate hydrochloride
- carbon dioxide
|
|
|
| |
| |
In general, anesthesia should first be induced in a tank and then followed by a smaller maintenance dose circulated over the gills during surgery. After surgery, fish must be revived before being released back into their environment. This period of post-operative care is important but is often disregarded by fish surgeons. |
|
|
| |
|
|
| |
|
|
| |
Suture materials
There are a wide variety of suture materials available for wound closure in fish surgery. Braided silk is one type of suture commonly used on fish for short-term studies, such as those performed in a laboratory. This type of suture is more prone to cause infection in fish because bacteria could easily get into the braids. However, monofilament (both absorbent and non-absorbent) sutures do not pose this problem. These types of suture (i.e., monofilament) are generally used on fish that are released back into the wild. They reduce tissue inflammation and thus promote wound healing for long-term studies. Sutures should be removed after the wound has healed but this may not be practical for field deployments. |
| |
|
|
The most common sizes of sutures used on small to medium bodied fish are 2/0, 3/0 and 4/0. Finer diameter sizes of suture (i.e., 4/0) must be used with care because they can act like a ligature and cut tissue. Larger diameter sizes (i.e., size 0, 1 and 2) of suture are used on larger fish, such as sturgeon, and have better retention.
In most instances, the preferred choice of suture needle is the cutting tip style over round needles. It is better and more convenient to use a suture kit with a strand of suture material already attached to the needle. This removes the complication of threading the strand into the needle during surgery and the needle/suture material is maintained in a sterile condition.
Suturing requires skill to be performed properly. The proper practice is to align opposing tissue after closing and then apply the appropriate amount of tension to make sure the wound is water tight and snug. A simple interrupted suture pattern is the most common type used in fish surgery and is the type that is discussed here. There are other suture patterns, such as simple continuous, horizontal mattress and continuous Ford interlocking used for skin closure that has had satisfactory results for fish. The first suture, for a simple interrupted suture pattern, should be placed in the midpoint of the wound and successive sutures (about 2 to 4 mm apart) are then worked alternately on each side until the wound is closed. Sometimes two layers of suturing are required. One on the inner muscular level with absorbable sutures prior to an external closure to minimize knot surface area and reduce drag. The two layers of suturing are commonly performed on larger species with fairly thick muscular wall.
Please also see paper on "Effects of suture material on incision healing, growth and survival of juvenile largemouth bass... ". |
|
|
| |
|
|
| |
Other forms of wound closure
Surgical staples or glue can also be used to close wounds. Although surgical staples can be used quickly and with minimal skill, the healing of incisions may not be definite and this can lead to transmitter loss. When staples are used, proper staple size (according to the organism) is important in preventing the tearing of tissue and in minimizing necrosis on the wound area. Wounds closed by glue (usually cyanoacrylate, i.e., crazy glue or vet bond) often tend to reopen resulting in substantial inflammation and tissue necrosis. Glue can be used in combination with suture for “locking” sutures into place, particularly when fish are benthic or engaged in reproduction when there is substantial abrasion against the region with sutures. |
|
|
| |
|
|
| |
|
|
| |
| Training to conduct fish surgery is a multi-step process that begins with reading, followed by observing trained individuals, then practice on non-animal models, followed by mentoring and hands on training with an experienced surgeon and a veterinarian to provide an assessment of skill and to correct problems. After this, the surgeon should continue to practice to maintain and improve their skill and proficiency. It is important that fish surgical practices be conducted at a professional level and to ensure that procedures are the least invasive to fish and will thus improve the quality of the resultant data. |
|
|
| |
The Cooke Lab is available to provide training workshops and other consultation related to fish surgery.
|
|
|
1125 Colonel By Drive, Ottawa, ON, K1S 5B6, (613) 520-2600 |
|
